Maintaining Food Safety Standards: A Comprehensive Guide to Listeria monocytogenes and Salmonella Control Compliance
Recent notable outbreaks of foodborne illnesses caused by Listeria monocytogenes and Salmonella spp. have prompted a proactive approach in searching for effective methods of eliminating pathogen risk. The value of sampling the production and processing environment is highlighted in European regulation EU2073/2005 to identify and prevent pathogens in food products.
Now, 18 years after the publication of this regulation, how far have we come to understand and mitigate the processing environment's risks?
Lack of Guidelines and Standardization in Food Processing Environment Monitoring
It goes without saying that clear guidelines and standardized practices are necessary for food processing environment monitoring. Article 5 of Regulation 2073/2005 delves into the specific rules for testing and sampling, shedding light on the expectations placed on food business operators. The regulation emphasizes the need for sampling processing areas and equipment to detect potential risks to public health, such as Listeria monocytogenes in ready-to-eat foods (RTE).
Sampling should be conducted using the Standard ISO 18593 as a reference method to ensure compliance with the set criteria. Notably, this standard was initially a technical specification but became a full ISO Standard in mid-2018. In Europe, official recommendations from organizations like eCDC and EFSA suggest identifying Critical Sampling Sites (CSS) to build an effective sampling scheme based on production days and sampling time.
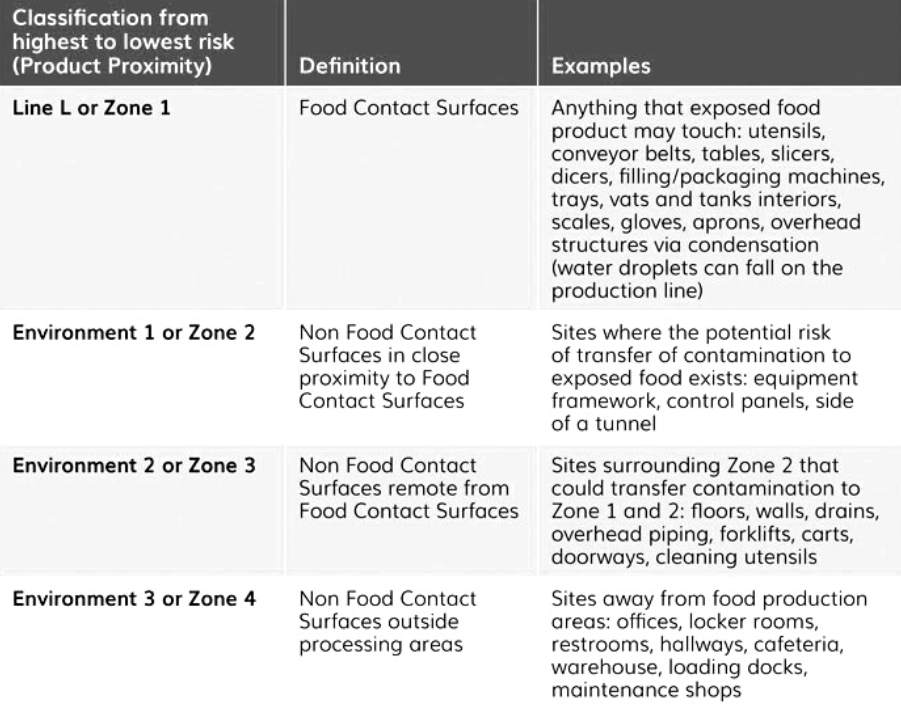
Figure 1 provides definitions and examples related to the proximity approach, distinguishing between Food Contact Surfaces (FCS) and non-Food Contact Surfaces (nFCS). The concept of proximity was first proposed by ICMSF in 2002, featuring a four-layer approach: one for FCS and three for nFCS. This approach has been adopted by the US Food and Drug Administration (FDA) and various US industry-based guidelines, including those from the Almond Board of California, GMA, and United Fresh Produce, using Zone 1 to Zone 4.
 Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
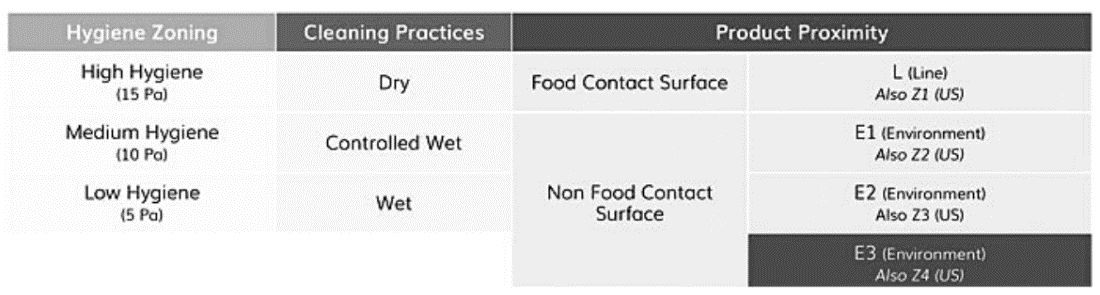
However, it is essential to note that there are often misconceptions about hygiene zoning classification. Contrary to popular belief, Zone 1 does not necessarily imply high hygiene, nor does Zone 4 indicate low hygiene. Some industry players have introduced an alternative classification using Line (L) for FCS and E1 to E3 for nFCS. European industry-based guidelines, such as the recent one from the “European Association of Fruit and Vegetable Processors” (PROFEL), also acknowledge and incorporate this approach. Regulatory authorities' official recognition of this approach is still an ongoing process.
Considerations for Sampling in Food Production Environments
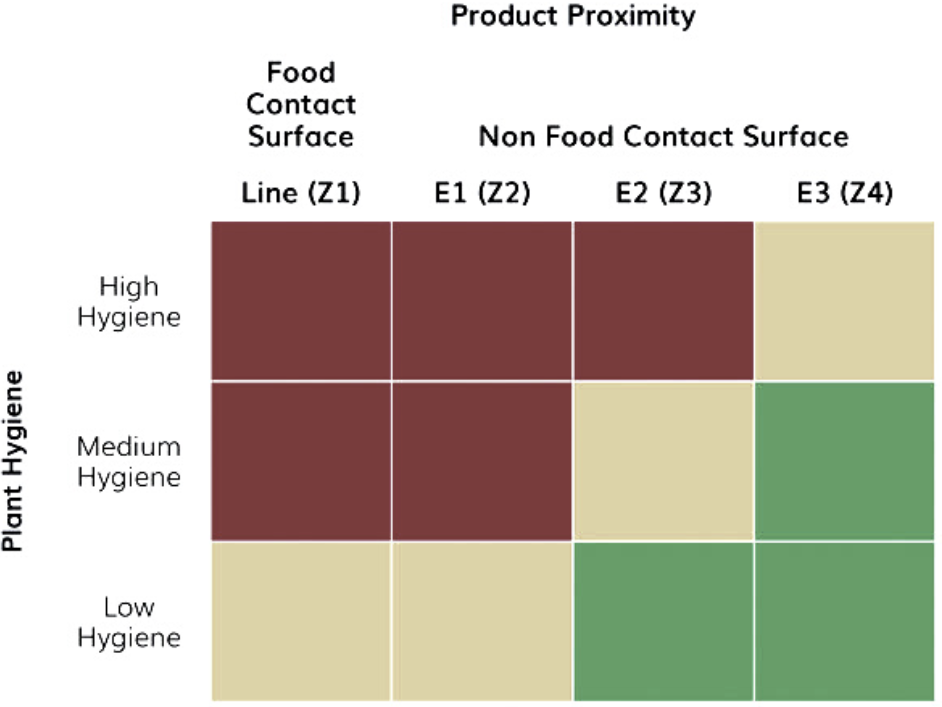
Three criteria should be considered when determining the location of sampling points within food production premises, as depicted in Figure 2: zoning based on hygiene levels, existing cleaning practices, and product proximity.
 Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
For instance, during the reception of raw milk through a tube hole, the area falls under Zone 1 (low hygiene), where wet cleaning is employed. In such cases, it raises whether it is meaningful to swab and search for Listeria in that specific location. Considering its position before pasteurization, would a positive result indicate a risk of food product contamination? Since sampling requires time and financial resources, prioritization becomes essential. Pathogen environment monitoring is often likened to a "seek and destroy" approach or a "bear hunt." This perspective is particularly relevant for investigation sampling points, as the objective is to isolate targeted pathogens and identify harborage niches. However, once these pathogens are identified, the question arises: What should be done next?
Strategies After Obtaining a Positive Swab Result
Following a positive swab result, subsequent samples are often referred to as "vector samples" by the FDA. Many industry-based guidelines recommend employing the "starburst" sampling technique in response to a positive finding. In this context, the proximity sampling approach proves highly valuable. Unless the product could be the source of contamination, a positive result should indicate how close the contamination is to the product.
If an E2/Z3 positive is obtained, further investigation is required to determine if nearby E1/Z2 surfaces have been contaminated. Additionally, it is crucial to assess whether the nearby E3/Z4 surface or other E2/Z3 points could be the source of contamination. At this stage, implementing a reinforced finished product sampling scheme is optional.
However, an E1/Z2 positive presents a more challenging scenario. It may be necessary to investigate L/Z1 areas and implement an increased finished product sampling scheme, which requires careful planning rather than improvisation. It is important to note that a positive result on an L/Z1 sample should be treated as a positive finished product testing. When sampling L/Z1, releasing the product when an analytical result is available is vital.
Food business operators should proactively establish a mitigation plan in anticipation of potential positive results rather than formulating one in response to such occurrences. In the event of obtaining a positive sample, it is advisable to reference the most recent negative sample collected from the same location and carefully evaluate any deviations that may have arisen since then. As a precautionary measure, suspending ongoing and preceding production activities may be necessary.
 Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
Img reference: https://www.newfoodmagazine.com/article/144453/processing-environment-monitoring/
Considering the potential impact on production, expedited time-to-result (TTR) alternative analysis methods should be prioritized over culture-based reference methods. Alternative methods should be appropriately validated according to the ISO16140-2 scheme.
Distinguishing Cleaning Monitoring from Processing Environment Monitoring
Considering recent outbreaks involving Listeria monocytogenes and Salmonella spp in Europe, there is now heightened attention from food business operators, regulators, analytical providers, and third-party laboratories to determine the best approach for monitoring.
It is essential to differentiate between cleaning and processing environment monitoring, as these are two distinct approaches. The ISO 18593 standard already emphasizes this distinction in its abstract: "This document does not apply to the validation of cleaning and disinfection procedures." Proper sampling timing plays a crucial role in ensuring that the focus is on monitoring the conditions of the processing environment rather than assessing the effectiveness of cleaning practices.
Time at which sampling should be performed is well written in the ANSES/EURL Lm “Guidelines on sampling the food processing era and equipment for the detection of Listeria monocytogenes”, Chapter 5:
“Therefore, to increase the probability of detecting a persistent strain, sampling should be performed during processing, after at least two hours of production or at the end of production runs, i.e. before cleaning and disinfection [4, 8-11]. In processing lines where food products are manufactured from raw products which are not submitted to a treatment that reduces the level of microorganisms (raw cheeses, for example), L. monocytogenes in a surface sample taken during the processing run may originate from these raw products as well as from the places where L. monocytogenes cells can persist in the food processing environment. In plants, where pasteurized products or raw materials not frequently contaminated are processed (pasteurized cheeses, for example), L. monocytogenes in a surface sample should be investigated as a persistent Listeria.”
It is no surprise that certain food producers establish their own guidelines regarding supplier quality expectations. While implementing suitable cleaning practices is undoubtedly essential, it is crucial to acknowledge that they alone cannot guarantee food safety. Comprehensive processing environment monitoring involves various aspects, such as zoning, hygienic design, and adherence to good manufacturing practices. These elements collectively contribute to ensuring the highest standards of food safety.
Conclusion: Ensuring Food Safety through Processing Environment Monitoring
Implementing monitoring measures for pathogens of concern and hygienic indicators in the processing environment is a concept that may seem straightforward, but its practical execution is complex. It requires careful deliberation to ensure that the monitoring efforts are directed toward the critical areas, supported by comprehensive preventative and corrective action plans.
During the selection of sampling sites, it is crucial to consider potential remedial options in advance. Will a positive result have implications for the safety of food production? Should a reinforced testing scheme be implemented? Additionally, it is essential to assess whether it is necessary to halt the release of finished products during investigative procedures. In the present landscape, food business operators must pay attention to the threats posed by harborage niches and resident pathogenic strains. Recognizing and addressing these risks is essential to maintain a high food safety standard throughout the industry.
In conclusion, processing environment monitoring is critical in ensuring food safety within the food industry. While appropriate cleaning practices are fundamental, they are not sufficient on their own. By implementing a comprehensive approach that encompasses zoning, hygienic design, and adherence to good manufacturing practices, food producers can establish a robust framework for maintaining optimal food safety standards.
References
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs
- ISO, 2018. ISO 18593: Microbiology of the food chain — Horizontal methods for surface sampling
- Codex Alimentarius Commission. Code of hygienic practice for low-moisture foods. CXC 75-2015. Adopted in 2015. Revised in 2016. Amended in 2018.
- Almond Board of California, 2010. Pathogen Environment Monitoring Program (PEM).
- GMA, 2014. Listeria monocytogenes Guidance on Environmental Monitoring and Corrective Actions in At-risk Foods
- United Fresh Produce, 2013. Guidance on Environmental Monitoring and Control of Listeria for the Fresh Produce Industry
- PROFEL, 2020. Hygienic guidelines for the control of Listeria monocytogenes in the production of quick-frozen vegetables
- BfR opinion No 004/2020 issued 20 January 2020. Escherichia coli in flour – sources, risks and prevention. DOI 10.17590/20200120-102303
- IDF, 2019. Ecology of Listeria spp. and Listeria monocytogenes; Significance in Dairy Production.
- ANSES/EURL Lm, Version 3-20/08/2012. “Guidelines on sampling the food processing aera and equipment for the detection of Listeria monocytogenes”

