The Reliable Alternative to Chromogenic Rapid Tests
Swiss company NEMIS Technologies AG has launched its innovative N-LightTM series, which is a drastic improvement compared to the classical chromogenic rapid tests used for environmental monitoring. This article highlights the key technical differences between the technologies.
The major advantage of environmental monitoring (EM), if performed rigorously, is the prevention of end product contamination by rigorous early process controls. Doing EM on-site screening kits enables users to test flexibly and frequently as rapid tests can be carried out by anyone, anytime. While laboratory-based genotypic solutions such as PCR are specific, relying on laboratory services can be costly and complex. Limited budgets impose significant constraints on the volume of samples that can be collected and analyzed, statistically reducing the likelihood of detecting pathogen hotspots.
Instead, rapid screening tests offer the opportunity for quality managers in food production sites to stay autonomous and internalize the knowledge obtained by a locally performed set of controls for the best risk management that fits its unique settings. Sadly, most rapid tests fall short in terms of specificity and sensitivity as competing microflora challenges the reliability of these methods.
Competing Microflora as a Challenge for the Specificity of Chromogenic Methods
Competing microflora can pose two risks to growth-based assays. First, enzymatic activities are used to detect Listeria monocytogenes with chromogenic methods as well as the N-LightTM luminescent test kit. Although PI-PLC, a virulence factor with high specificity, is used in several detection methods (also in ISO ALOA plates), rare virulent bacteria (e.g., L. ivanovii, pathogenic staphylococci) can produce this enzyme.
Read also: The 3 Most Popular Misconceptions about Environmental Monitoring
Second, Listeria innocua and Enterococcus spp. can outcompete Listeria monocytogenes in both broths and plates and further reduce reliability and performance. Fortunately, a carefully selected cocktail of highly specific bacteriophages and antibiotics within the NEMIS proprietary enrichment broth effectively eliminates the most troublesome competing microflora. The competitive study below shows that the N-LightTM test significantly outperforms other chromogenic tests by this smart manipulation within the growth phase. This NEMIS advantage may be particularly significant when the samples are taken from surfaces where product residues and competing microflora are present.
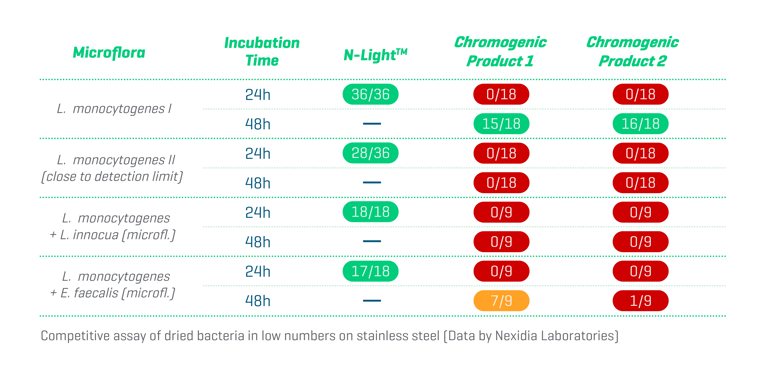 Validation Study according to the AOAC Performance Tested Methods SM
Validation Study according to the AOAC Performance Tested Methods SM
The N-LightTM L. monocytogenes kit was able to detect all 52 strains, representing 12 serotypes, tested during the inclusivity study. Moreover, N-LightTM L. monocytogenes did not detect any of the 30 non-Listeria monocytogenes in the exclusivity strain set, which included other Listeria spp. The specificity of the kit was therefore validated according to the exclusivity study. Concerning the matrix study on stainless steel without competitive microflora, the NEMIS method detected significantly more positive samples than the reference method for the low inoculation, particularly for stainless steel and ceramics.
Read also: The need for self-control programs concerning Listeria monocytogenes
If you have any questions, directly reach out to NEMIS’ customer service, who is at your disposal at any time: techsupport@nemistech.com.

