Rapid testing methods for E.coli monitoring in food production
Ensuring food safety is a critical concern in the food production industry. Contaminated food products can pose health risks to consumers, resulting in product recalls and damage to brand reputation. In this article, we will provide an overview of E. coli and its risks in food production and highlight the importance of rapid testing methods for effective monitoring.
Overview of E. coli and Its Risks in Food Production
The origin of E. coli in nearly all food and water contamination cases can be attributed to exposure to fecal contamination, which can occur through multiple sources, such as farms where cattle carry E. coli in their gut. In addition, wildlife, such as deer, birds, and pests in agricultural areas, can extract feces containing E. coli on plants, leading to potential contamination.
Raw milk can be contaminated with E. coli from cattle feces during milking. Furthermore, during cattle slaughter, E. coli from wastes can occasionally contaminate beef, typically on the surface of the meat.
Cooking can often eliminate the bacteria in many cuts of beef, such as steak. Ground meat, however, poses a higher risk as the grinding process can mix and distribute the contaminated meat throughout the product, making thorough cooking essential.
Poor personal hygiene can also be a potential source of fecal contamination. For instance, failure to wash hands properly after using the toilet can transfer harmful E. coli from infected food handlers to food products, leading to potential contamination.
Traditional methods for E. coli testing in food production often involve time-consuming and laboratory-intensive processes that must be carried out in a specially equipped microbiology laboratory. Often incubation of samples for 24-48 hours is required before results can be obtained.
Rapid, lab-free detection of E. coli with a novel NEMIS N-LightTM test
The novel N-Light™ E. coli test is a rapid qualitative method for detecting Escherichia coli as a hygiene indicator in food processing. This test can monitor and control factors such as compliance with hand hygiene rules, hygiene status of incoming materials (e.g., potential contamination with manure residues, etc.), and effectiveness of sterilization steps in food processing. It is suitable for use in food processing areas and equipment as part of an environmental monitoring program and for testing process water and other liquid samples. The E.coli rapid test was designed to be user-friendly and versatile, serving as a hygiene indicator for various settings, including factory surfaces, tools, production machines, and employee hand hygiene. Unlike agar plates and complex sample preparation, this test offers a convenient solution, delivering reliable results within 16 hours.
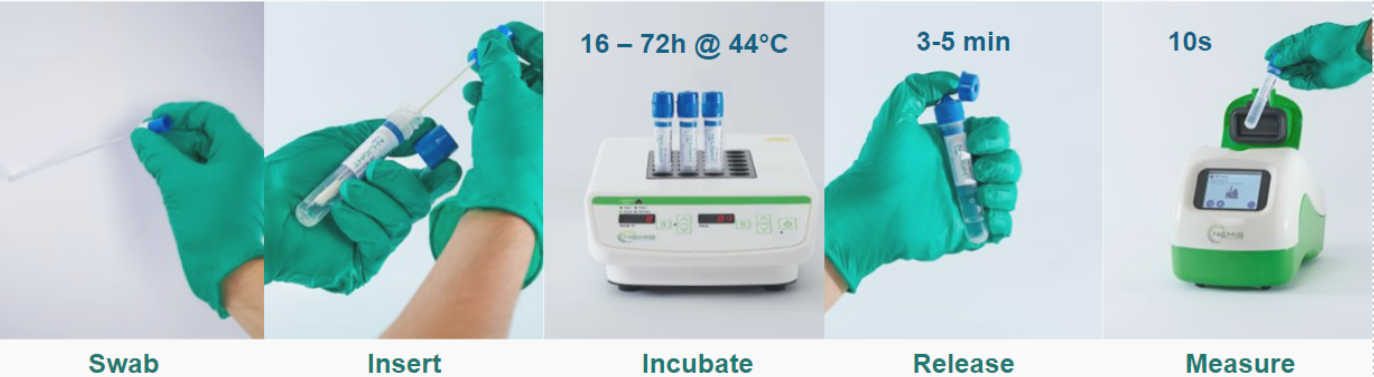
The advantages of the N-Light™ E. coli test are:
- Lab-free testing
- Take action after only 16 hours.
- Anyone can do it
- Closed system from sampling to detection
- Digital results in 4 simple steps
Other detection methods for E. coli used in the food industry:
Polymerase Chain Reaction (PCR)
PCR is a molecular-based technique that amplifies the DNA of E. coli in food samples, allowing for highly sensitive and specific detection of this bacteria. PCR needs enrichment of bacteria before analysis, typically requiring an incubation step of 16 to 24 h in the laboratory. DNA extraction involves several working steps to ensure reproducible and reliable results. PCR requires personnel specifically trained in molecular biology methods and a set of instruments (incubator, micropipettes, laminar flow bench, centrifuge, thermocycler, and DNA electrophoresis equipment or qPCR device).
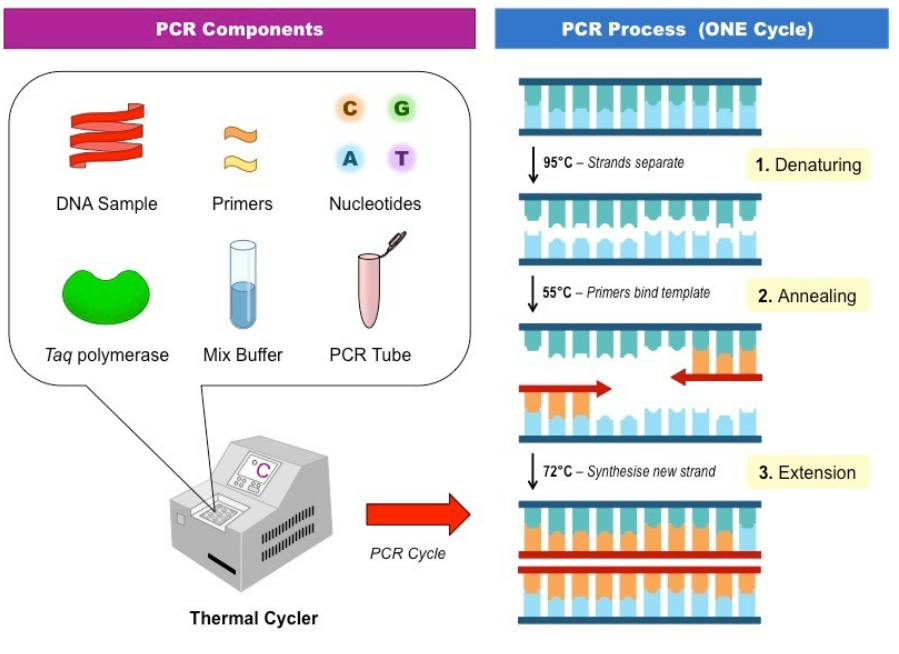
Solid Phase Cytometry
E.coli can be detected using solid-phase cytometry (SPC) in combination with fluorescent viability staining. In this method, water samples containing E. coli are filtered through a polyester membrane with a pore size of 0.4 µm under vacuum to capture the bacteria. The retained E. coli on the filter is then treated with reagents to induce the enzyme β-D-glucuronidase and incubated at 37⁰C for 3 hours. The induced cells are then labelled, with only viable cells able to mark the enzyme, causing the bacteria to cleave the non-fluorescent substrate and leaving only the fluorescent end inside the viable cells.
The advantages of Solid Phase Cytometry for E. coli detection are:
- Ability to detect viable organisms, providing a more accurate assessment of bacterial presence.
- Relatively low cost per test, making it cost-effective for routine testing.
- Rapid and visual detection of contaminated water using the ScanRDI® device.
The limitations of Solid Phase Cytometry for E. coli detection are:
- Limited to detecting viable organisms only and may not detect non-viable or dormant cells.
- Reliance on fluorescent signal, which may have limitations in sensitivity and specificity.
- Involves a multi-step process, requiring careful handling and coordination of different steps.
- Additional steps, such as biochemical tests or mass spectrometry, may be needed to confirm E.coli presence.
Viable Cell Counting
There are various methods to measure bacterial concentrations, and a straightforward approach is viable cell counting on agar plates. In this method, the bacterial sample is serially diluted (1:10, 1:100, 1:1000, etc.) in sterile water or Phosphate Buffer Saline (PBS) and plated on semi-selective media for isolation of Escherichia coli, e.g. chromogenic agar plates containing synthetic enzyme substrate X-Glucuronide. The plates are incubated overnight at 37°C. Further biochemical tests or MALDI-TOF may be performed on pure isolated colonies to confirm the presence of E. coli and differentiate it from other organisms.
The advantages of viable cell counting on agar plates are:
- Ability to detect viable organisms, providing a direct measure of culturable bacterial concentration.
- Relatively low cost per test, making it cost-effective for routine testing.
The limitations of viable cell counting on agar plates are:
- Limited to detecting viable organisms that can grow on the chosen media may not capture non-culturable or slow-growing bacteria.
- Reliance on colony formation, which may have limitations in sensitivity and specificity.
- Involves a multi-step process, requiring dilution, plating, and incubation steps.
- Additional steps, such as biochemical tests or mass spectrometry, may be needed to confirm E. coli presence.
References:
- https://starfishmedical.com/blog/best-method-detect-e-coli/
- http://ib.bioninja.com.au/standard-level/topic-3-genetics/35-genetic-modification-and/pcr.html
- https://www.researchgate.net/publication/42388104_Detection_of_Escherichia_coli_O157H7
- https://link.springer.com/content/pdf/10.1007/s00253-009-1879-x.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/11777581
- https://courses.lumenlearning.com/boundless-microbiology/chapter/counting-bacteria/

